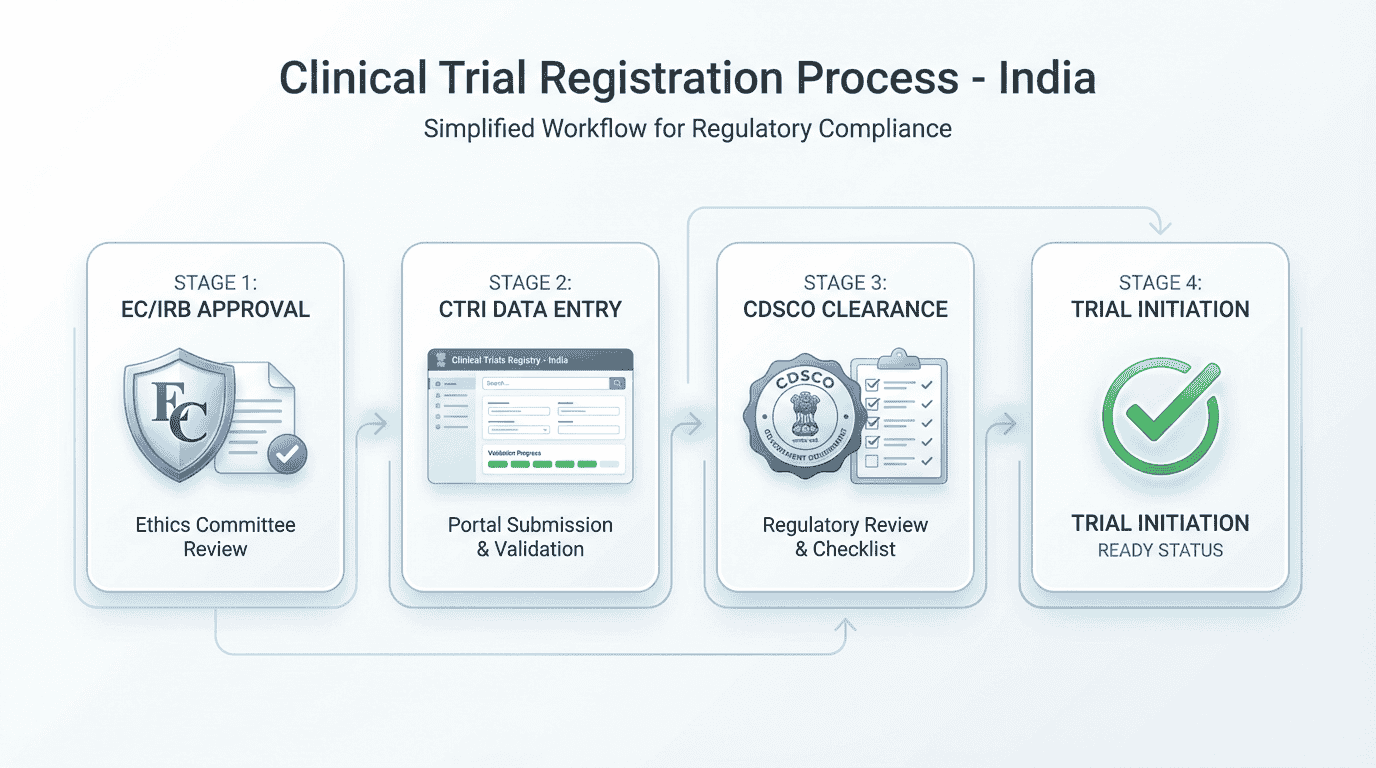
Clinical trial registration India involves a clear, regulated sequence that every sponsor must follow before enrolling participants. Under the New Drugs and Clinical Trials Rules (NDCT Rules), 2019, registration ensures transparency, participant protection, ethical oversight, and compliance with national and global publication standards. The steps below explain the complete process in simple, structured terms.
Strengthen early trial readiness with cleaner pre-screening workflows and organized participant intake through DecenTrialz
Why Clinical Trial Registration Is Required
Registration ensures:
- Transparency of study methods and objectives
- Participant safety and ethical oversight
- Prevention of duplicate studies
- Compliance with WHO and ICMJE journal requirements
- Public availability of essential trial information
Trials registered late may face publication barriers, regulatory concerns, or EC objections.
Key Authorities in the Registration Process
CTRI (Clinical Trials Registry–India)
Public registry for prospective trial registration.
Assigns a REF number immediately upon submission, and a CTRI Number after full review and approval.
Ethics Committee (EC/IRB)
Ensures participant rights and safety.
A dated EC approval letter is mandatory at CTRI submission.
CDSCO and DCGI
India’s national regulatory authority responsible for approving trials involving new drugs, regulated medical devices, and certain high-risk interventions.
Step-by-Step Registration Process for Sponsors
The steps below reflect NDCT Rules 2019, updated CDSCO processes, current CTRI expectations, and SUGAM portal requirements.
Step 1: Prepare the Clinical Trial Protocol
The protocol is the foundational document describing:
- Study purpose and design
- Eligibility criteria
- Number of participants
- Interventions
- Visit schedules and assessments
- Safety oversight strategy
- Data analysis plan
- Insurance and compensation provisions
Sponsor tasks:
- Finalize protocol with version number and date
- Ensure consistency across informed consent forms, case report forms, and all supporting documents
Step 2: Prepare Participant Documents
Participant-facing documents must be clear and easy to understand.
These include:
- Participant Information Sheet (PIS)
- Informed Consent Form (ICF)
- Translated versions (if required)
These documents must explain:
- Purpose
- Procedures
- Risks and benefits
- Voluntary nature of participation
- Privacy protection
- Compensation for study-related injury
Step 3: Submit the Study to the Ethics Committee (EC/IRB)
The EC/IRB reviews:
- Protocol
- PIS/ICF
- Investigator’s Brochure
- Case report forms
- Site suitability details
- Investigator CVs
- Insurance and compensation documents
Regulation Update
Under NDCT Rule 19(5), EC review may occur in parallel with CDSCO review, but:
- CTRI requires dated EC approval letters at the time of submission, and
- CDSCO issues final permission only after EC approval.
Timeline: Commonly 2–6 weeks, depending on EC schedules.
Sponsor tasks:
- Ensure EC approval letters contain correct protocol title, version, site name, and approval date
- Maintain approvals for all participating sites
Step 4: Determine Whether CDSCO Approval Is Required
A corrected and accurate decision table for 2025:
| Scenario | CDSCO Approval Required? | Notes |
| New drug / Investigational New Drug (IND) | ✔ Yes | Requires Form CT-04 via SUGAM |
| Global clinical trial where product is IND or regulated | ✔ Yes | IND/global new drug studies require CDSCO approval |
| Global trial using an approved, marketed drug without new claims | ✖ Often Not Required | Purely post-marketing observational global studies may be exempt |
| Bioavailability / Bioequivalence (BA/BE) study of regulated drug | ✔ Yes | 45-day review timeline |
| Medical device trial (regulated categories) | ✔ Yes | As per device risk class |
| Pure observational study with no intervention | ✖ Not Required | CTRI still encourages registration when unclear |
CDSCO Submission Includes:
- Application via SUGAM Portal
- Form CT-04 (for permission to conduct a clinical trial)
- Fee payment through Bharatkosh
- Protocol + Investigator’s Brochure
- CMC and safety data (if applicable)
- Preclinical or prior clinical data
- EC approval before final permission
- PI and site information
- Compensation and insurance documentation
CDSCO Review Timelines (NDCT Rules)
- New drug clinical trials: Up to 90 days
- BA/BE studies: Up to 45 days
Sponsor tasks:
- Register on SUGAM with Digital Signature Certificate
- Upload documents in required formats
- Respond promptly to CDSCO queries
Step 5: Collect All CTRI-Required Information
Information needed for CTRI:
- Study titles
- Health condition
- Study type and design
- Phase of study
- Inclusion/exclusion criteria
- Objectives and outcome measures
- Sample size (total + per site)
- Recruitment timelines
- PI and site details
- Dated EC approval letters
- CDSCO approval details (if applicable)
- Sponsorship details
Documents needed:
- EC approval letter(s) (dated and signed)
- CDSCO approval letter (if required)
- Final protocol
- PIS and ICF
- Investigator CVs
- Insurance and compensation documents
Step 6: Create a CTRI Account
Actions include:
- Register on ctri.nic.in
- Provide PI or responsible contact details
- Verify email
- Access the online registration form
No fee is charged.
Step 7: Complete the CTRI Registration Form
CTRI requires precise, consistent information.
Important expectations:
- Titles must match EC approval exactly
- Site list must match EC-approved sites
- Study phase must reflect protocol and regulatory approvals
- Interventions must be described clearly without promotional wording
- Recruitment dates must be realistic and consistent
- Observational studies with even minimal intervention elements must still register
CTRI Timeline
- REF number assigned immediately after submission
- Review usually starts within 10 working days
- CTRI number assigned after clarifications and approval
Sponsor tasks:
- Conduct a complete internal quality check
- Fix inconsistencies in names, dates, or versions
- Respond quickly to CTRI clarifications
Step 8: Post-Approval Responsibilities
Once approved:
- CTRI number becomes public
- Enrollment can begin (if CDSCO and EC approvals are active)
Updating CTRI
Updates require contacting CTRI at ctri@gov.in to unlock the record.
Updates needed for:
- Adding or removing sites
- Changing investigators
- Amendments to protocol
- Recruitment status updates
- Timeline extensions
Additional Sponsor Responsibilities (NDCT Rules)
- Report Serious Adverse Events (SAEs) within 14 days to EC and CDSCO
- Maintain insurance coverage and compensation compliance
- Ensure all amendments receive EC approval
Expected Timelines
| Step | Expected Timeline |
| Protocol preparation | ~1–3 weeks |
| EC/IRB approval | ~2–6 weeks |
| CDSCO approval (new drugs) | Up to 90 days |
| CDSCO approval (BA/BE) | Up to 45 days |
| CTRI review | ~10 days initial + time for clarifications |
Common Mistakes Sponsors Should Avoid
- Submitting CTRI form without dated EC approval letters
- Assuming global trials always require CDSCO approval
- Using different protocol titles across EC, CDSCO, and CTRI
- Selecting incorrect study design or phase
- Uploading outdated protocol or ICF versions
- Entering site names not listed in EC approval
- Delayed responses to CTRI or CDSCO clarifications
- Failing to update CTRI after amendments
Avoiding these mistakes prevents delays and ensures smooth regulatory compliance.
Final Summary: A Clean Sponsor Roadmap
- Finalize protocol and participant documents
- Submit to EC for approval (parallel CDSCO review allowed under Rule 19(5))
- Determine CDSCO need using the corrected IND-based criteria
- Submit Form CT-04 via SUGAM if required
- Gather all CTRI-required documents including dated EC letters
- Create CTRI account
- Submit CTRI form and receive REF number
- Respond to CTRI clarifications and receive CTRI number
- Update CTRI through email unlock requests when needed
This end-to-end process creates a fully compliant pathway for clinical trial registration in India, ensuring ethical conduct, regulatory readiness, and participant protection.
DecenTrialz: Supporting Early Trial Readiness
DecenTrialz does not file EC submissions, CDSCO applications, or CTRI registrations.
Those responsibilities remain with the sponsor.
However, many registration challenges come from:
- Inconsistent eligibility criteria
- Unclear participant-facing materials
- Misaligned workflows between protocol and operations
- Poor documentation structure
- High screen failure rates due to unclear pre-screening
DecenTrialz strengthens early trial readiness by helping sponsors:
- Translate protocol eligibility into structured digital pre-screeners
- Ensure participant-facing materials are consistent
- Reduce mismatches between protocol text and operational workflows
- Organize participant information for cleaner site review
- Deliver only pre-qualified participants once recruitment begins
This improves study startup efficiency and prevents avoidable screen failures.
Improve Trial Readiness
Ensuring clear eligibility criteria and well-organized participant workflows makes the EC, CDSCO, and CTRI process far smoother.
Enhance your trial readiness with DecenTrialz:
Learn More: www.decentrialz.com
Contact the Team: www.decentrialz.com/contact
Leave a Reply