Clinical trials are essential for advancing medicine. They are how doctors and researchers discover better ways to prevent, diagnose, and treat illnesses. Many of the treatments we depend on today, such as vaccines and cancer drugs, exist because volunteers took part in clinical trials.
If you have ever wondered what clinical trials involve, how they are designed, or whether joining one might be right for you, this article is a beginner’s guide that explains the basics in clear and simple terms.
What Are Clinical Trials?
Clinical trials are research studies that test whether a new medical approach, such as a drug, device, or therapy, is safe and effective for people. Researchers follow strict rules to measure how well a treatment works, monitor side effects, and protect the health of participants. A treatment can only be approved for public use after passing through these steps.
Clinical trials help with:
- Testing new treatments before they become widely available.
- Comparing existing treatments to see which works best.
- Understanding how different groups of people respond to the same treatment.
People choose to join trials for many reasons. Some hope to improve their own health, while others want to contribute to medical progress. Many say volunteering gives them a sense of purpose, knowing their involvement may help future patients.
How Safety Is Protected
Before a trial begins, it is reviewed by an independent ethics committee called an Institutional Review Board (IRB). The IRB ensures that the study is ethical, fair, and designed to protect participants.
Every participant must also provide informed consent. This means you will receive clear information about the study’s purpose, potential risks, expected benefits, and what participation involves. Only after reviewing this information and asking questions can you decide whether to join. Signing the consent form does not commit you permanently. You are free to leave the trial at any time.
Privacy is also protected. Clinical trials in the United States must follow laws such as HIPAA, which safeguard your personal health information.
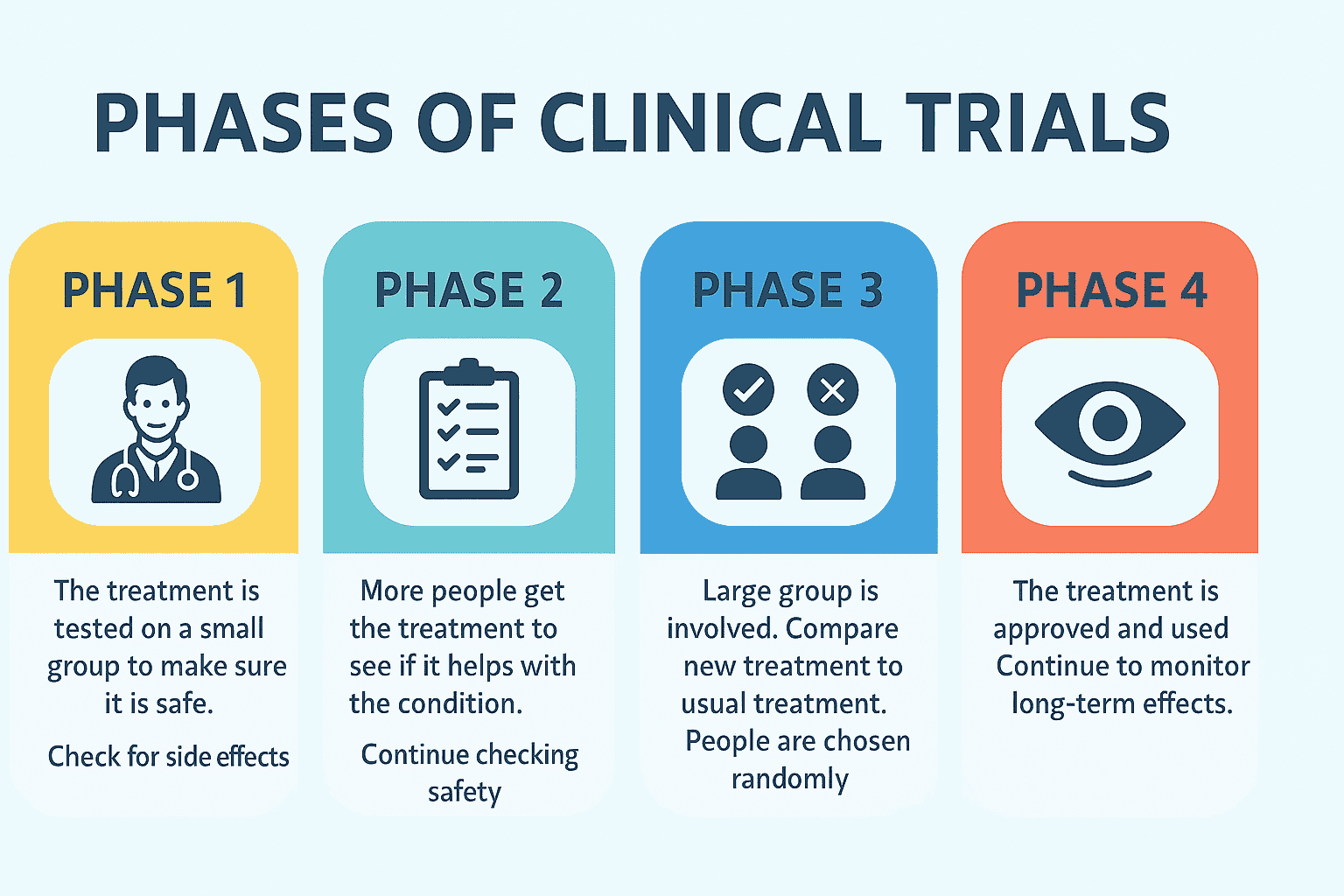
The Phases of Clinical Trials
Trials are usually conducted in stages, known as trial phases. Each phase answers different questions and involves different numbers of participants.
Phase 1: First-in-Human Testing
- Involves about 10 to 30 volunteers.
- Focuses on safety and finding the right dose.
- Doctors closely monitor participants for side effects and how the body reacts.
Phase 2: Testing Effectiveness
- Involves 100 or more participants.
- Examines whether the treatment works for the condition.
- Safety continues to be monitored, and researchers look for early signs of improvement.
Phase 3: Large-Scale Comparison
- Involves hundreds or even thousands of participants.
- Compares the new treatment to standard care or a placebo.
- Participants are randomly assigned to groups to keep results fair.
- Often conducted as double-blind, meaning neither patients nor doctors know who is receiving which treatment until the study ends.
Phase 4: Ongoing Monitoring
- Conducted after a treatment has been approved and made available to the public.
- Tracks effectiveness in larger, more diverse populations.
- Identifies long-term or rare side effects.
How Clinical Trials Are Designed
Each clinical trial follows a detailed plan called a protocol. This document explains the study’s purpose, who can join, what treatments will be tested, how long the study will last, and how safety will be monitored.
The IRB reviews the protocol before the trial begins to ensure participant protection. Trials must also comply with privacy rules such as HIPAA.
Once approved, a research team led by a principal investigator oversees the study. This team often includes physicians, nurses, and coordinators who:
- Recruit participants and explain the study.
- Collect informed consent.
- Monitor participants’ health.
- Record data throughout the study.
Some modern trials use decentralized or hybrid approaches. This means that not all activities happen at the hospital or clinic. For example, participants might attend telehealth visits, use wearables or apps to send health data from home, or receive study medication by delivery. These approaches make participation easier, especially for people who live far from research centers.
Who Can Participate in a Clinical Trial?
Not everyone qualifies for every trial. Eligibility is determined by criteria such as:
- Age and gender.
- Type and stage of a disease.
- Previous treatments.
- General health.
For example, one cancer trial may accept only patients with a specific tumor type, while a diabetes trial may have blood sugar requirements.
Diversity is also important. Researchers want trials to reflect real-world populations, so they aim to include people of different ages, races, and ethnicities. This ensures treatments are safe and effective for everyone.
If you are interested, the research team will review your medical history and conduct tests to see if you qualify. If you do, you will then review and sign an informed consent form. Remember, participation is voluntary and you can leave the study whenever you choose.
How to Find a Clinical Trial
If you would like to explore clinical trials, here are common ways to start:
- Talk with your doctor. They may know about trials related to your condition.
- Search online. The U.S. government maintains a public database at ClinicalTrials.gov, where you can find thousands of ongoing studies.
- Use a trial finder. For example, DecenTrialz provides a tool to search by location and condition.
- Check patient advocacy groups. Organizations focused on conditions such as cancer or diabetes often share trial opportunities.
When you find a trial, read its summary carefully, speak with the study contact, and discuss it with your doctor. They can help you decide if it is the right choice for you.
Why Clinical Trials Matter for Patients
Clinical trials are the foundation of medical progress. They make it possible to develop treatments that are safer, more effective, and more personalized.
For participants, a trial can offer:
- Access to expert medical care.
- Early access to treatments not yet available to the public.
- The chance to contribute to discoveries that could benefit others.
Most importantly, clinical trials provide hope. Each volunteer helps move science forward and supports a healthier future. By choosing to participate, you are helping yourself and making a difference for patients everywhere.