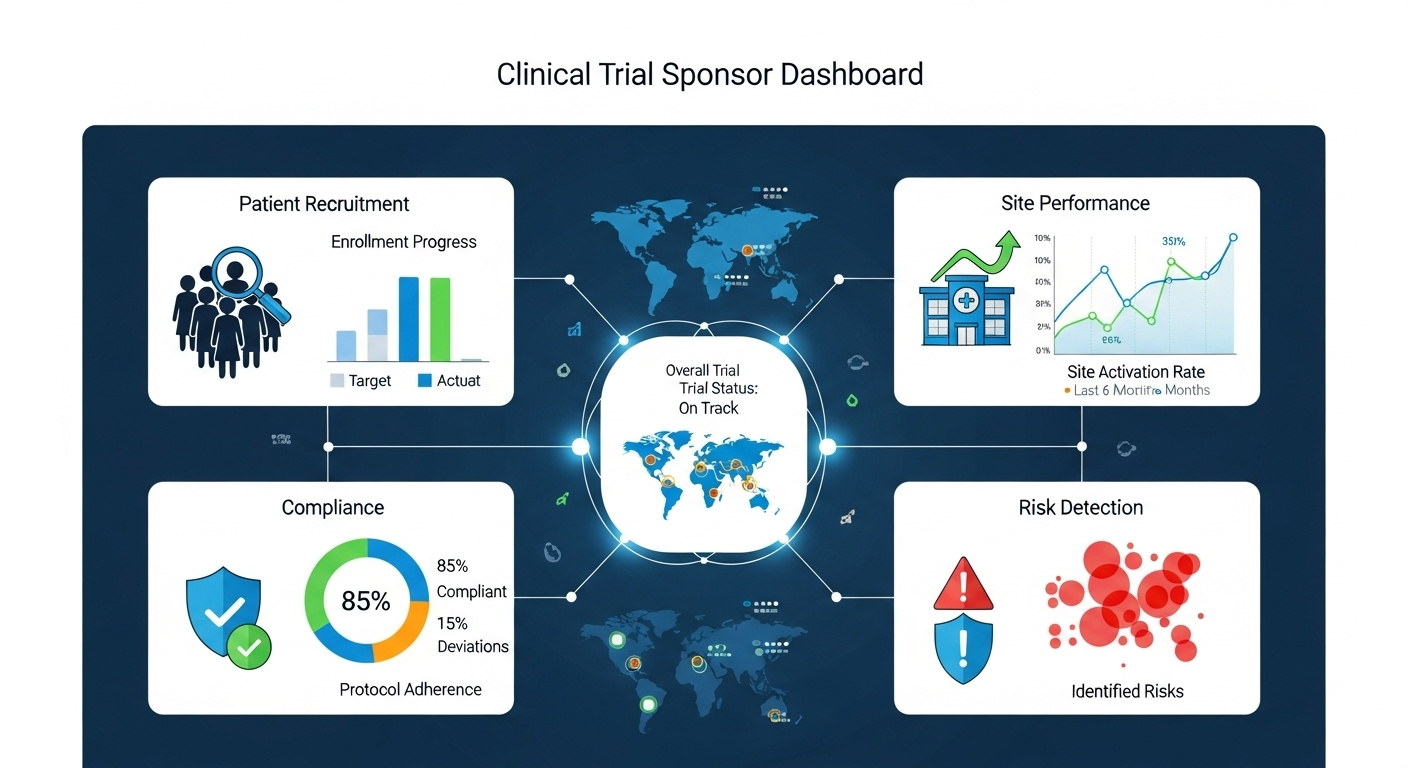
Imagine this: you are sitting in a review meeting, surrounded by reports from different sites. Recruitment numbers are already weeks old. A dropout spike that happened last month is only now reaching your desk. A critical site missed its enrollment target, but you find out after the milestone is already gone.
This is not mismanagement. It is the reality of relying on traditional systems that give you information too late to act. In clinical research, those delays cost not just money, but also opportunities for patients and for innovation.
This is where sponsor dashboards in clinical trials change the story. By bringing real-time insights into your hands, dashboards give you the ability to spot issues the moment they happen, track progress as it unfolds, and make confident, data-driven decisions that keep your trials on course.
1. Why Traditional CTMS Leaves Sponsors Waiting
For years, Clinical Trial Management Systems (CTMS) have been the backbone of trial operations. They are structured, necessary, and valuable. But for oversight, they often fall short:
- Data comes late: Reports are static and lag behind reality.
- Sites feel disconnected: Sponsors see pieces, not the whole picture.
- Manual processes create risk: Updates take time, and errors slip in.
- Sponsors stay reactive: Problems surface only after they become costly.
In fast-moving, global trials, “better late than never” is not good enough.
2. What Exactly Are Sponsor Dashboards?
A sponsor dashboard is not another system to manage. It is a window into your trials in real time.
Instead of waiting for end-of-month reports, sponsors can open a dashboard and immediately see:
- Current enrollment across sites
- Site activation timelines and data entry speed
- Compliance trends and early risk indicators
Dashboards bring oversight to life, showing you not only the current status of your trials but also where they are headed next.
3. Benefits of Dashboards for Trial Oversight
The power of dashboards is not in the technology alone. It’s in how they make oversight more practical and human for sponsors:
- Clarity in recruitment: Track enrollment daily, monitor diversity, and see dropout risks before they escalate.
- Confidence in site performance: Spot which sites are leading and which need extra support.
- Early risk detection: Identify protocol deviations or slow data entry before they snowball.
- Actionable visuals: Numbers transform into patterns you can act on, not just tables you skim.
This is oversight that finally works the way sponsors always wanted it to.
4. Dashboards as Data-Driven Sponsor Tools
Dashboards don’t just tell you what happened. They help guide what you should do next.
- Predictive forecasting: See whether recruitment will hit the target on time.
- Dynamic KPIs: Measure trial health continuously instead of quarterly.
- Resource allocation: Adjust budgets or CRO support while the trial is still running, not after it is too late.
One sponsor used a dashboard to spot lagging enrollment mid-trial. By reallocating outreach to higher-performing sites, they recovered weeks of lost time. That type of agility is impossible with static CTMS reports.
5. Oversight Becomes Transparent and Collaborative
Transparency builds trust. With dashboards, everyone in the ecosystem : sponsors, CROs, and investigators works from the same version of the truth.
Sponsors no longer have to wonder, “Is this data current?” Instead, they can engage sites with confidence, improve accountability, and strengthen relationships that are often strained by fragmented reporting.
6. What Sponsors Should Look For
Before investing, sponsors should ask:
- Does the solution meet HIPAA and ICH-GCP standards?
- Is patient privacy fully protected?
- Will it integrate smoothly with CTMS and EDC?
- Does the vendor have real-world experience with global trials?
The right dashboard is not just a tool, but a partner in trial success.
7. The Reality of Adoption
Adopting dashboards does take change. Teams must learn new habits, budgets need planning, and leaders must push past the “we’ve always done it this way” mindset. But sponsors who make the leap gain oversight that feels natural, not forced.
8. Why the Time to Act Is Now
Clinical trials are becoming more global, more data-rich, and more competitive. Sponsors who adopt dashboards today gain speed, transparency, and stronger recruitment outcomes. Those who delay will watch competitors set the new industry standard.
The choice is clear: remain reactive, or lead with real-time, data-driven oversight.
Dashboards are rewriting the sponsor experience in clinical trials. They move oversight from delayed reporting to immediate insight, from fragmented data to connected teams, from reactive decisions to proactive leadership.
Sponsors who embrace dashboards now will not only manage trials more effectively, but also lead the way in shaping the future of research.
In today’s environment, dashboards are no longer optional. They are the new standard for sponsor oversight.