When Awareness Becomes Personal
Colorectal cancer clinical trials are shaping the future of prevention, early detection, and treatment at a time when colorectal cancer remains one of the most commonly diagnosed cancers in the United States.
On a quiet Tuesday morning in March, Maria sat at her kitchen table holding a pathology report she never expected to receive. Just weeks earlier, life felt normal. Now she was facing decisions about surgery, chemotherapy, and what would come next.
She is not alone. According to the American Cancer Society’s 2026 Colorectal Cancer Facts & Figures, an estimated 154,270 new colorectal cancer cases will be diagnosed in the United States this year. For many families, March is no longer just the start of spring. It becomes the beginning of questions, uncertainty, and hope.
March is officially National Colorectal Cancer Awareness Month, recognized by Presidential Proclamation since 2000 to encourage education, screening, and research participation. Each year, the first Friday of March is observed as Dress in Blue Day, a nationwide awareness event amplified by the Colorectal Cancer Alliance and covered widely in the media.
The Centers for Disease Control and Prevention reports that colorectal cancer remains one of the leading causes of cancer-related death in the United States, despite being one of the most preventable and treatable cancers when detected early. At the same time, the National Cancer Institute maintains hundreds of active colorectal cancer clinical trials aimed at improving treatment precision, reducing side effects, and expanding options for patients.
For patients like Maria, and for those who have never participated in research before, awareness month is not just symbolic. It can be a moment to explore something many people overlook: colorectal cancer clinical trials.
PURPOSE: A First Look at Clinical Research
If you have never joined a study before, the phrase clinical trial may sound intimidating. Some people worry about being treated like an experiment.
In reality, today’s colorectal cancer clinical trials are carefully designed studies that follow strict safety protocols. Many focus on improving existing treatments, reducing unnecessary therapy, or expanding access to promising innovations.
Below are three active and accessible colon cancer clinical trials currently enrolling in the United States. Each represents a different approach to improving care and each is explained in plain language.
TRIAL 1 – ctDNA-Guided Adjuvant Chemotherapy
Could a Blood Test Help You Avoid Unnecessary Chemotherapy?
Sponsor: National Cancer Institute supported cooperative research groups
What It Tests: Circulating tumor DNA, or ctDNA, to determine whether chemotherapy is needed after surgery
Who It’s For: Patients with Stage II to III colorectal cancer following tumor removal surgery
Locations: Multiple academic and community cancer centers across the United States
After surgery, many patients receive chemotherapy as an added precaution. The goal is to eliminate microscopic cancer cells that may remain. But not everyone benefits equally from chemotherapy, and it can carry significant side effects.
This study uses a liquid biopsy blood test to detect circulating tumor DNA in the bloodstream. If no tumor DNA is detected, some patients may safely avoid chemotherapy.
Why It Stands Out
- Focuses on reducing overtreatment
- Uses personalized monitoring rather than a one size fits all approach
- Could minimize side effects such as fatigue and nerve damage
The American Society of Clinical Oncology has highlighted ctDNA research as one of the most promising tools in precision colon cancer research.
What It Could Mean for Patients
For first time participants, this trial may offer the possibility of skipping chemotherapy if the test shows it is unlikely to help, while still receiving close monitoring.
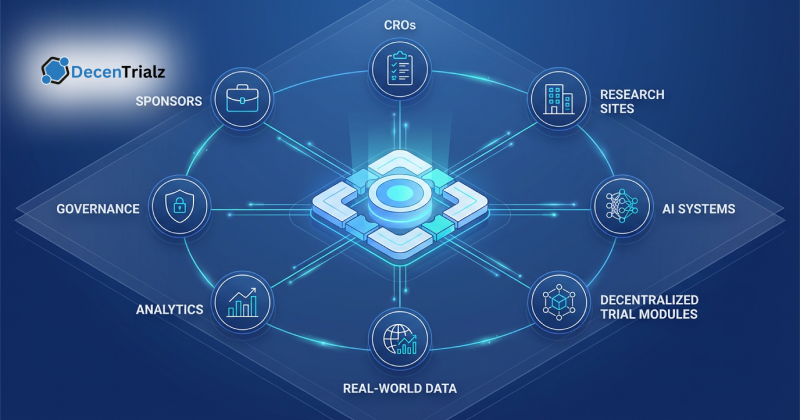
You can explore similar colorectal cancer clinical trials on the DecenTrialz platform to review available research opportunities.
TRIAL 2 – Pembrolizumab + Novel Agent for MSI-H Colorectal Cancer
Strengthening an Already Proven Immunotherapy
Sponsor: Merck in collaboration with National Cancer Institute sites
What It Tests: Pembrolizumab, an FDA approved immunotherapy, combined with a new immune enhancing drug
Who It’s For: Patients with MSI-H, or mismatch repair deficient, colorectal cancer
Locations: Oncology centers nationwide
Some colorectal cancers have a genetic feature called MSI-H, often associated with Lynch syndrome. Research published in PubMed Central has shown that patients with Lynch syndrome benefit from tailored surveillance and targeted therapies.
Pembrolizumab is already approved for MSI-H colorectal cancer. This study tests whether combining it with another immune targeting medication improves response rates.
Why It Stands Out
- Builds on an already established, FDA approved treatment
- Focuses on a clearly defined genetic subtype
- Aims to improve effectiveness without starting from the beginning
What It Could Mean for Patients
For eligible patients, this combination may enhance tumor shrinkage or extend remission. For first time volunteers, this type of study can feel less intimidating because it builds on a therapy already in use.
Find active studies and review trial details on DecenTrialz.
TRIAL 3 – CAR-T Targeting GUCY2C
Training Your Immune System to Recognize Colon Cancer
Sponsor: Academic medical centers with biotechnology collaborators
What It Tests: CAR-T cells engineered to target GUCY2C, a protein commonly found on colorectal cancer cells
Who It’s For: Patients with advanced or metastatic colorectal cancer
Locations: Select specialized U.S. cancer centers
CAR-T therapy involves collecting a patient’s own immune cells, modifying them in a laboratory to recognize cancer cells, and infusing them back into the body.
This study targets GUCY2C, a protein often overexpressed in colorectal cancer. It is considered an early stage clinical trial, meaning its primary goals are to evaluate safety and understand how well this approach may work in solid tumors such as colorectal cancer.
Why It Stands Out
- Highly personalized therapy
- Represents cutting edge colon cancer research
- Explores new options when standard treatments have stopped working
What It Could Mean for Patients
For patients with metastatic disease, this may offer access to next generation immunotherapy. At the same time, early phase trials focus first on safety and careful monitoring.
A Quick Word About Eligibility and Safety
Clinical trials are not right for everyone. Eligibility depends on your exact cancer stage, prior treatments, overall health, and personal preferences.
The most important step is to discuss any trial you are considering with your oncology team. They can help determine whether participation fits your medical situation and treatment goals.
How DecenTrialz Helps First Time Volunteers Navigate Options
Finding colorectal cancer clinical trials on your own can feel overwhelming. Trial descriptions often include medical terminology, eligibility criteria, and location details that are hard to interpret.
DecenTrialz helps patients filter trials by location, stage, eligibility, and treatment type in plain language, then bring a short list back to their oncology team to review together.
Ready to take your first step in clinical research? DecenTrialz makes it easy to find colorectal cancer trials near you in plain language, so you can review your options and discuss them with your oncology team. If you are not ready to participate yet, you can sign up for our volunteer registry to stay informed about future studies.
Awareness is the Beginning. Informed Action Is the Next Step.
National Colorectal Cancer Awareness Month reminds us that statistics represent real people. Behind the projected 154,270 new diagnoses this year are families making decisions about treatment, quality of life, and hope.
Clinical research has helped improve survival rates and expand treatment options over the past decades. Yet many eligible patients never explore trials simply because they do not realize they are an option.
March is a time to wear blue, talk about screening, and share stories. But it can also be a time to ask a new question: could exploring colorectal cancer clinical trials open another path forward for you or someone you love?